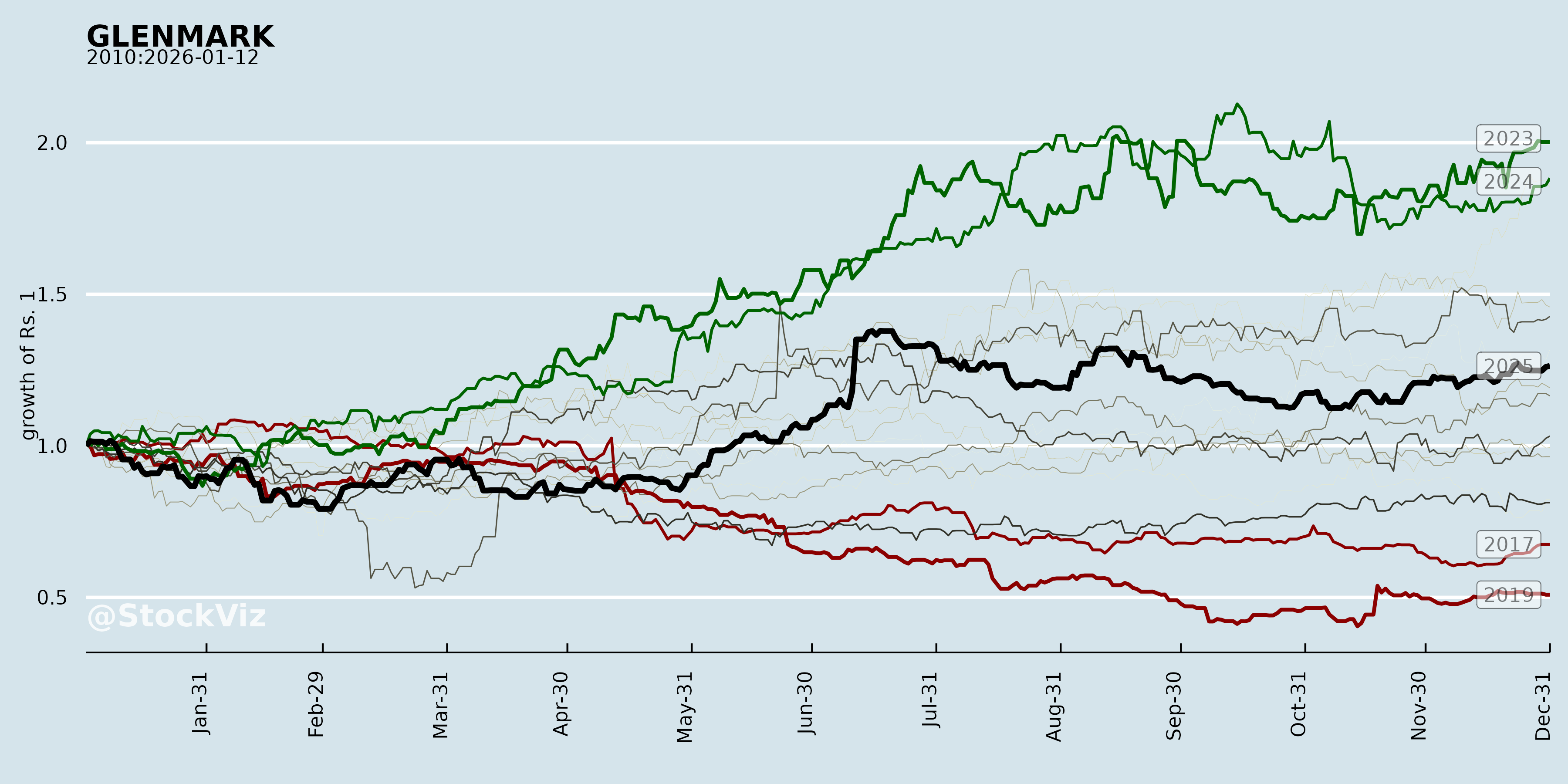
GLENMARK
Equity Metrics
January 13, 2026
Glenmark Pharmaceuticals Limited
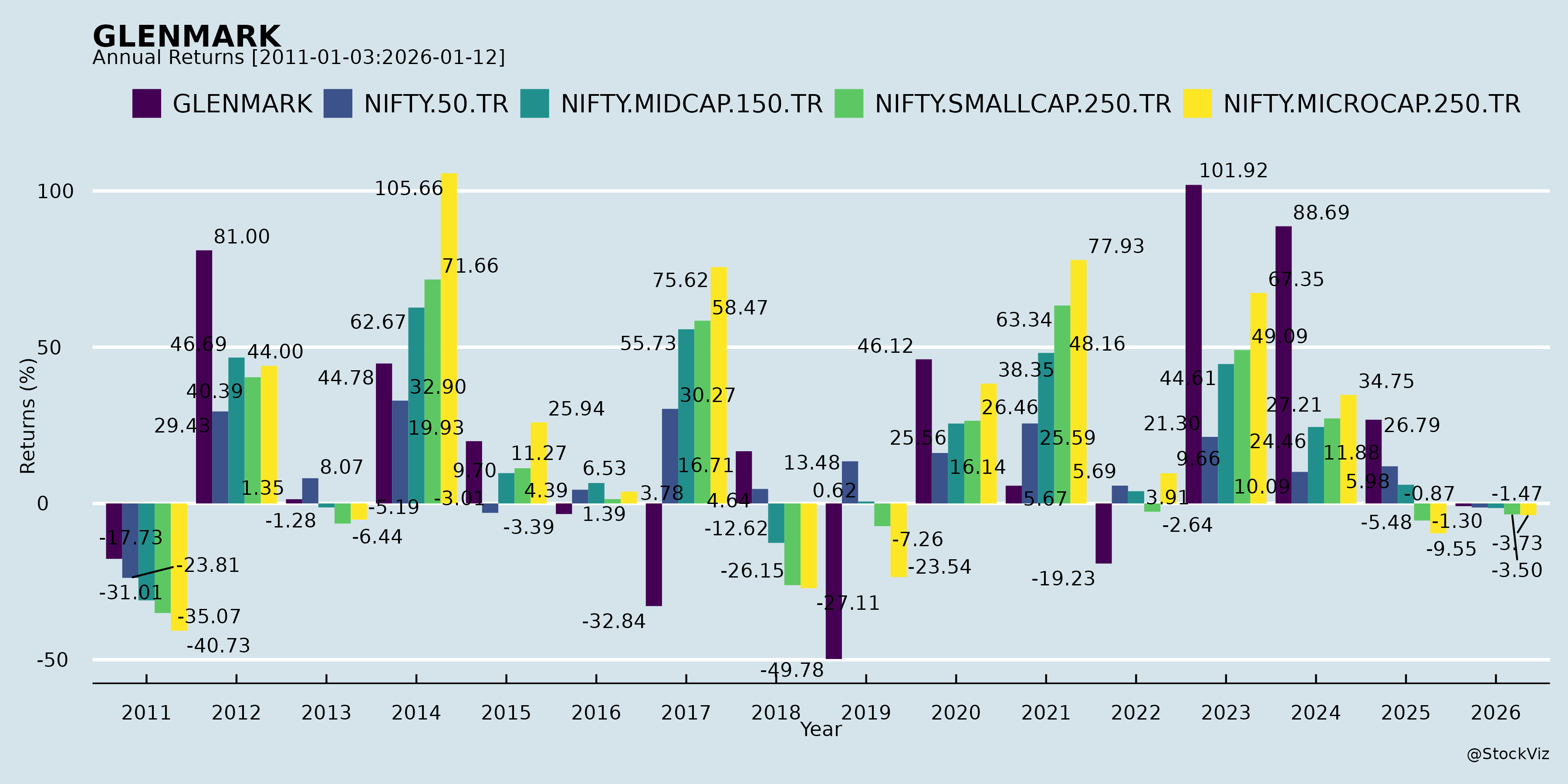
Annual Returns
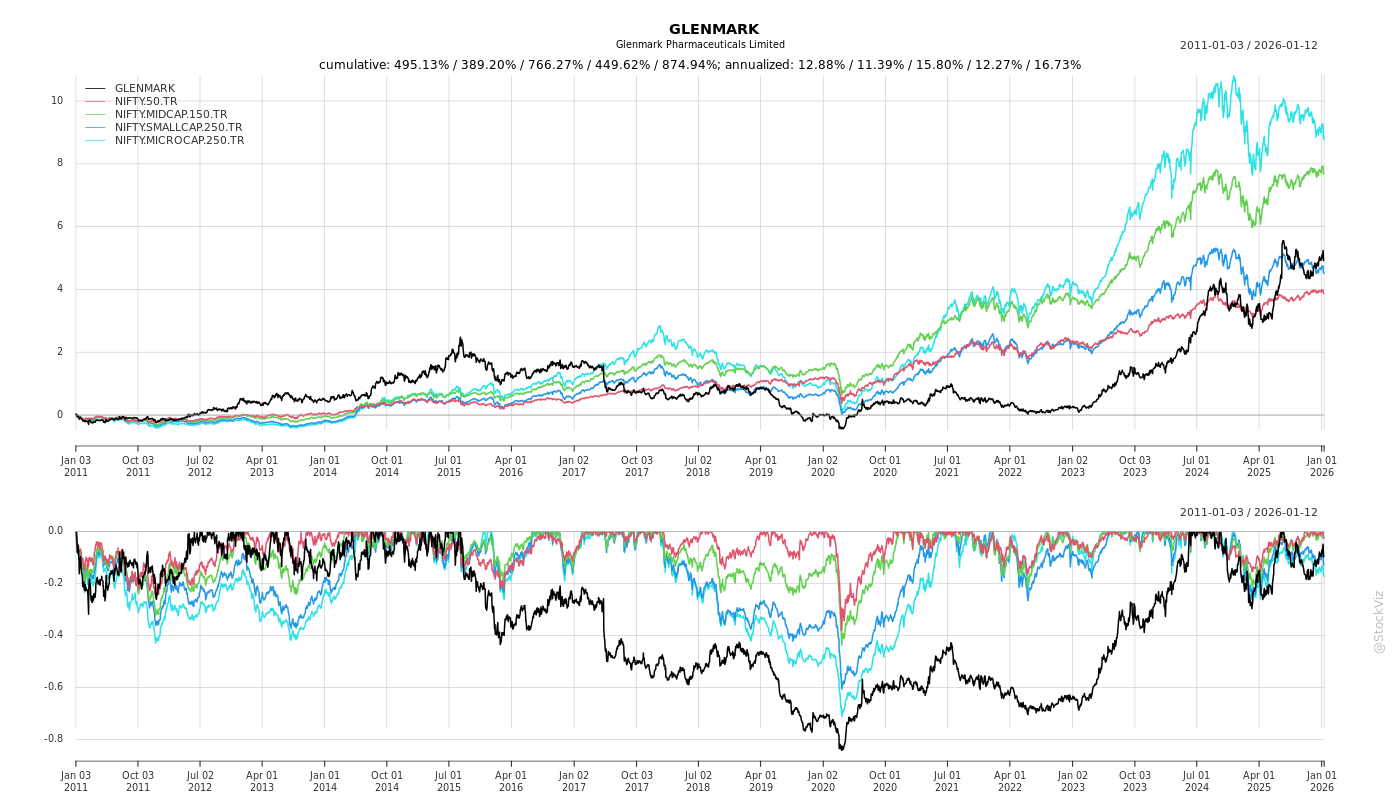
Cumulative Returns and Drawdowns
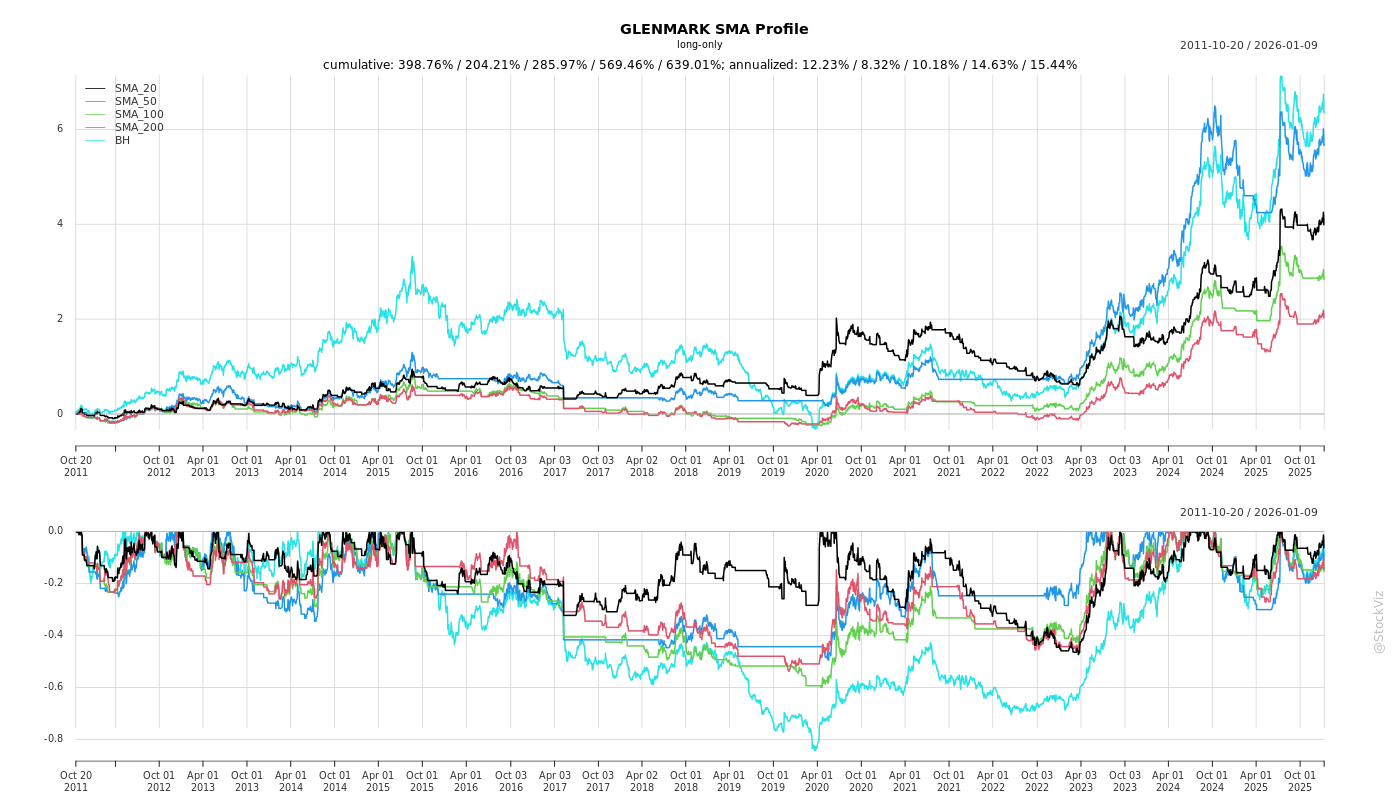
Fundamentals
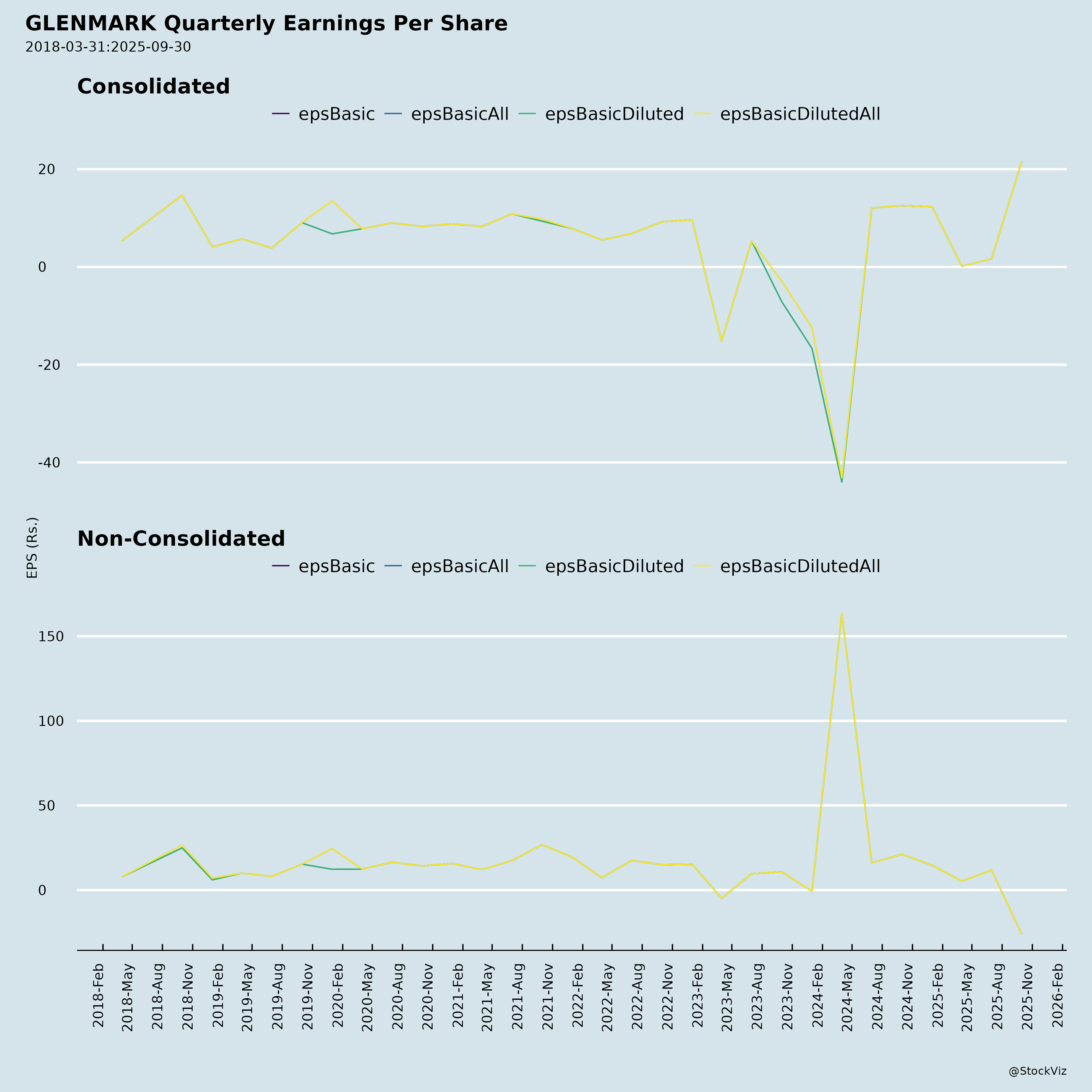
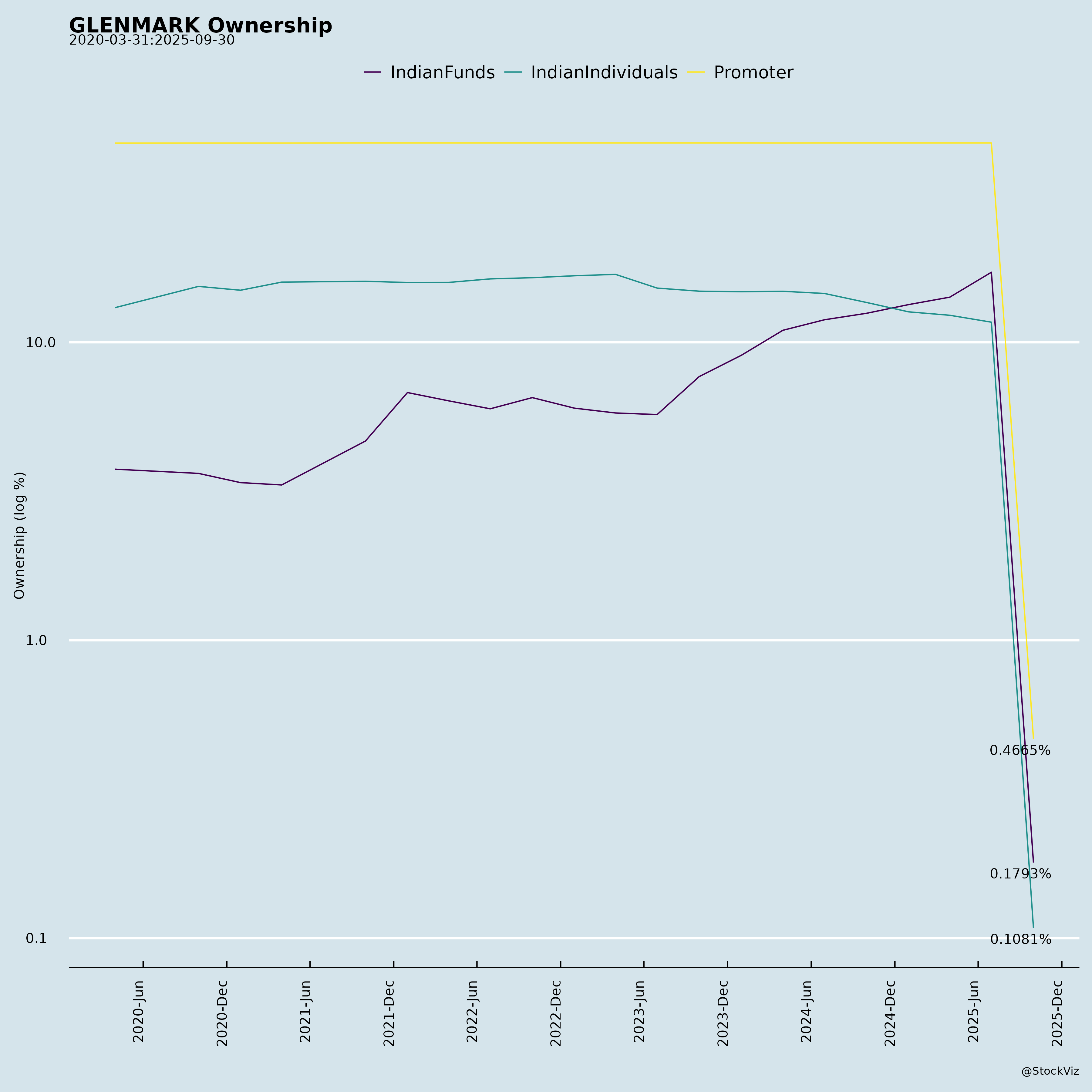
Ownership
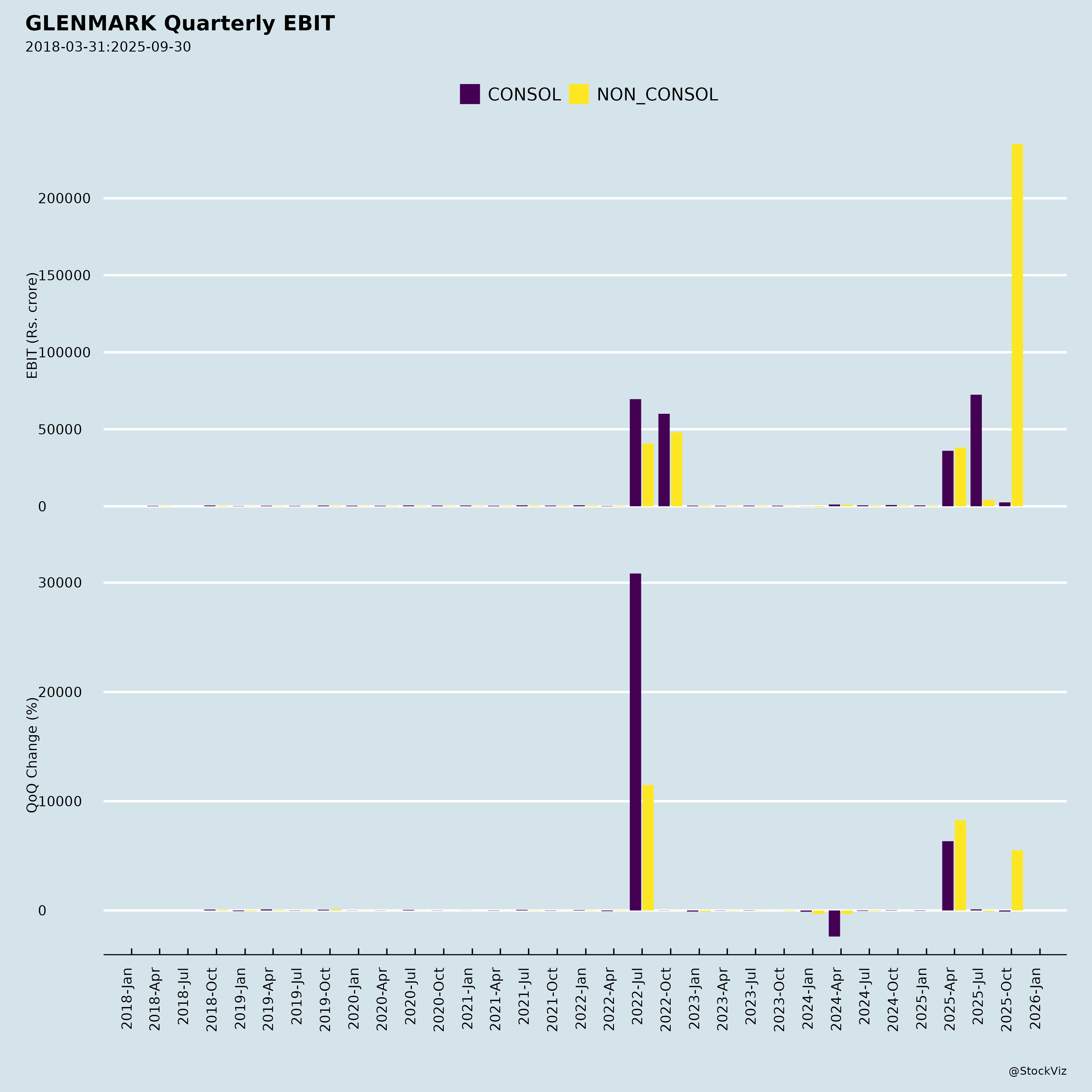
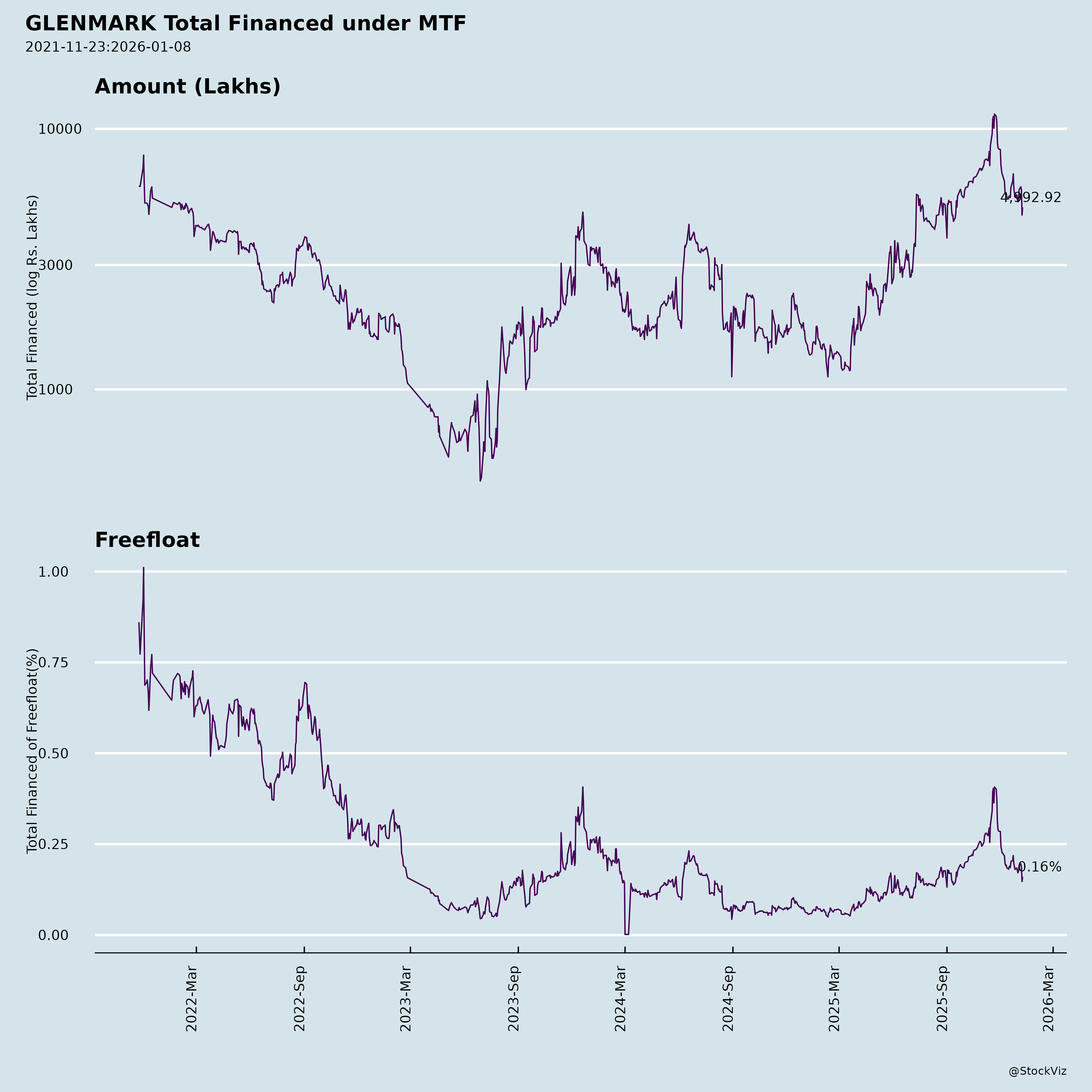
Margined
AI Summary
asof: 2025-12-08
Based on a comprehensive analysis of the provided financial disclosures, earnings report, auditor’s review, and press releases from Glenmark Pharmaceuticals Limited (GLENMARK – Scrip Code: 532296) as of November–December 2025, the following structured summary outlines the headwinds, tailwinds, growth prospects, and key risks for the company:
🔍 Company Overview
- Segment: Pharmaceuticals (Generics, APIs, OTC, Specialty)
- Operations: Global (India, US, Europe, Emerging Markets)
- Segments: One reportable segment – Pharmaceuticals
- ESG Focus: ESG committee reconstituted with strong leadership (ex-Cabinet Secretary Mr. P.K. Sinha as Chair)
- R&D Focus: IGI Therapeutics SA (innovation arm)
- Listed on: BSE & NSE
✅ TAILWINDS (Positive Catalysts)
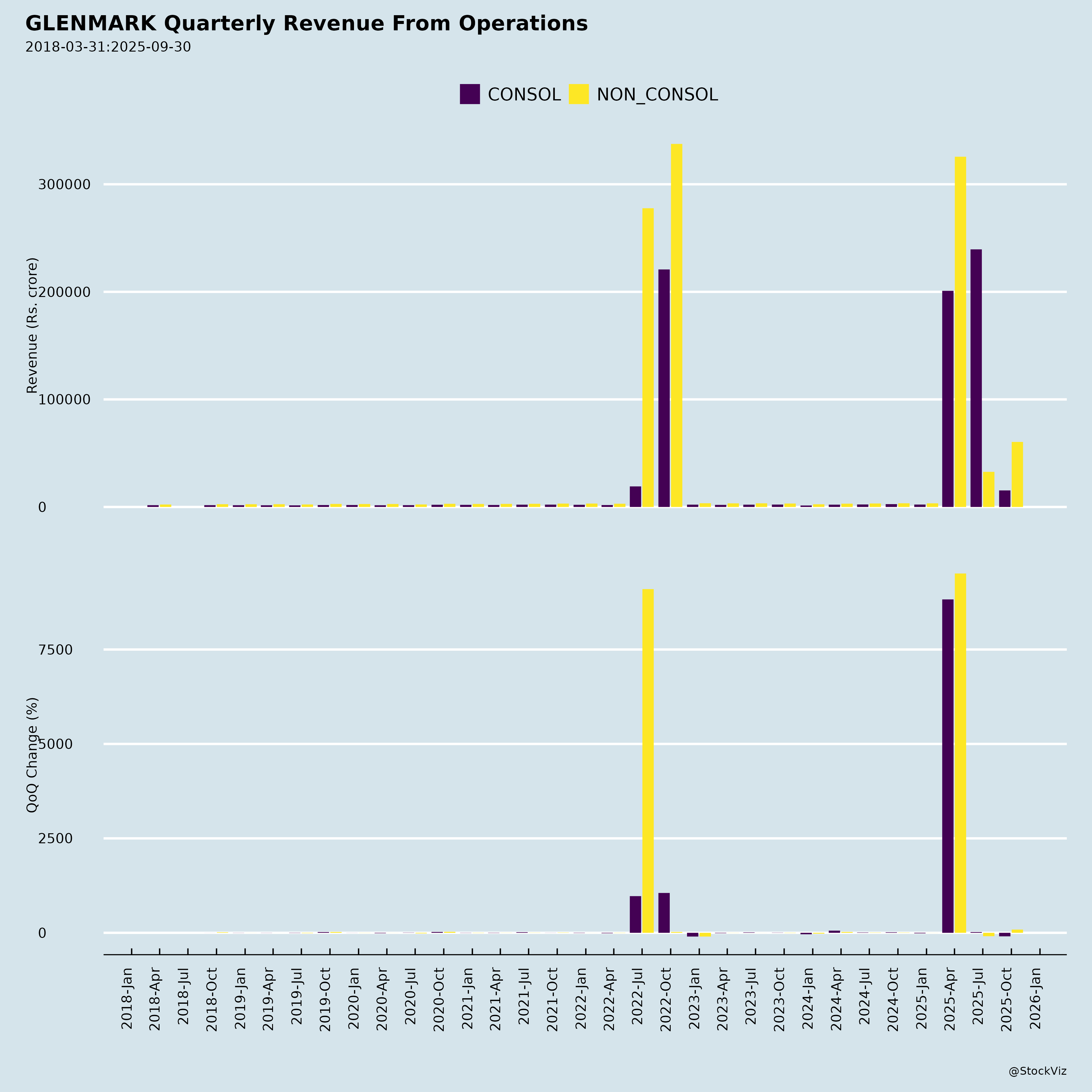
1. Strong One-Off Revenue Driver: AbbVie Licensing Deal
- $700M upfront payment from AbbVie for exclusive rights to develop, manufacture, and commercialize ISB 2001 in major markets (North America, Europe, Japan, Greater China).
- $525 million recognized as revenue in Q2 FY26 (July–Sept 2025), providing a significant short-term top-line boost.
- This is a high-margin, non-dilutive capital inflection point — rare in Indian pharma — signaling confidence in Glenmark’s R&D pipeline.
✅ Impact: Large cash inflow, improves balance sheet, enhances valuation, funds future R&D.
2. FDA Regulatory Recovery – Monroe (USA) Facility Cleared
- Monroe, NC facility received EIR with Voluntary Action Indicated (VAI) status from U.S. FDA (Nov 2025) after June 2025 inspection.
- Ends nearly 2.5-year Warning Letter (since June 2023), allowing resumption of commercial manufacturing.
- Prior Form 483 had 5 observations; clearance indicates regulatory compliance improvement.
✅ Impact: Restores supply chain reliability, regains credibility with US generics clients, supports revenue growth.
3. Zero FDA 483 Observations at Aurangabad Facility
- Successful Pre-Approval Inspection (PAI) at Chhatrapati Sambhajinagar (Aurangabad) facility with zero observations.
- Increases likelihood of product approvals in the pipeline and reflects strong operational compliance.
✅ Impact: Regulatory tailwind; strengthens portfolio approval momentum in US generic market.
4. Positive Cash Flow Recovery (Operating Level)
- Net cash from operating activities (Consolidated, H1 FY26): ₹28,356 crore
- Marked improvement vs. H1 FY25: (–₹3,635 crore)
- Adjusted for large one-offs, underlying operations show operational recovery.
✅ Impact: Strong underlying working capital management improvement.
5. Profitability Turnaround (Ex-Ethics) in Standalone
- Despite extraordinary charges, base profitability recovering:
- PBT before exceptional items: ₹7,498 crore (Half-year, standalone, up 5.3% YoY)
- Core operations stable amid pricing pressures.
✅ Impact: Foundational strength in business model persists.
6. Dividend Payout Credibility
- Interim dividend of ₹2.50/share (₹705.5 crore) approved.
- Signals confidence in liquidity and shareholder returns.
❌ HEADWINDS (Challenges & Drags)
1. Massive Exceptional Charges (Litigation Reserves)
Q2 FY26 Charges:
- Standalone & Consolidated exceptional loss: ₹9,318 crore (Q2) / ₹1,792 crore (H1 vs. prior H1)
- Key components:
- ₹9,318 crore: New U.S. antitrust settlement with United Healthcare Services Inc. (last opt-out plaintiff): ₹976 crore provision + associated costs.
- ₹5,901 crore: Inventory write-offs post GST 2.0 and new inventory management model.
- ₹4,885 crore: Receivables & current asset provisions.
- ₹2,089 crore: PPE impairment.
❌ Impacts net profit:
- Standalone PAT Q2: (₹7,392 crore) loss vs. ₹3,302 crore profit Q1
- EPS (Basic): ₹(26.19) down from ₹11.70
⚠️ Note: These are non-recurring but extremely heavy; may skew investor perception.
2. Impaired US Litigation Cloud
- Multiple antitrust suits in the U.S. (generic drug pricing allegations).
- Total settlements across FY24–FY26 exceed $132.25 million (~₹10,000 crore+).
- While company denies wrongdoing and cites “commercial settlement”, continuing reputational risk exists.
❌ Lingering ESG/litigation perception risk, especially in Western markets.
3. Restructuring in IGI Therapeutics
- IGI TSA restructured & manufacturing unit in Le-Chaux-de-Fonds (Switzerland) shut down.
- Total exceptional cost: ₹1,978 crore (FY25).
- CMC (Chemistry, Manufacturing, Controls) transitioned to CDMO — long-term cost control, but near-term disruption.
❌ Signal of pipeline prioritization; non-core R&D scaled back.
4. Decline in Indian & Emerging Market Revenue
- Domestic Formulations Growth Stagnant: Not highlighted as a driver; core Indian generic market likely facing pricing erosion and competition.
- Exports: U.S. segment not explicitly growing — likely under pressure from generic pricing declines.
5. High Leverage & Funding Needs
- Total Financial Liabilities (Standalone): ₹24,923 crore
- Net debt: Not directly stated, but significant lease and borrowing liabilities.
- No equity fundraising disclosed, but large one-time cash inflow from AbbVie helps.
❌ Refinancing risk in medium term if earnings remain volatile.
📈 GROWTH PROSPECTS
| Driver | Outlook |
|---|---|
| R&D Monetization | High potential – AbbVie deal opens door for future licensing. ISB 2001 likely an immuno-oncology/biologic asset. Pipeline focus may shift toward innovation. |
| U.S. Generic Recovery | Improving – Monroe facility restart and successful PAI at Aurangabad indicate better regulatory compliance. New product approvals likely to resume. |
| Emerging Markets & APIs | Stable revenue streams; not expanding but resilient. |
| ESG & Sustainability Credibility | SBTi approval strengthens ESG profile — advantage in global institutional investing. |
| Consumer Care Spin-off Completed | Transferred to GCCL (slump sale for ₹2,400 cr); non-material revenue, but improves strategic focus on core pharma. |
🔮 Potential Re-rating Drivers: - Sustained FDA compliance - Repeat deals from IGI TSA pipeline - Cost rationalization post restructuring
⚠️ KEY RISKS
- Regulatory Risk (FDA Exposure):
- Despite progress, past Warning Letter history makes U.S. business vulnerable; another quality lapse could trigger backtracking.
- Litigation Risk:
- U.S. antitrust suits may not be fully resolved; new private lawsuits possible. Ongoing need for large provisions.
- Pipeline Risk for IGI TSA:
- After AbbVie deal and restructuring, future R&D output may shrink unless new assets show promise.
- Inventory & Working Capital Volatility:
- ₹5,901 crore inventory provision signals legacy model inefficiencies — GST 2.0 digitization may hit margins short-term.
- Currency & Global Macroeconomic Risk:
- ~40%+ revenue from international markets — exposure to INR appreciation, supply chain inflation, trade barriers.
- Generic Price Erosion in US/EU:
- Industry-wide headwind; Glenmark has limited pricing power in commoditized markets.
🔚 SUMMARY: Investment Thesis – Neutral to Positive
| Parameter | Assessment |
|---|---|
| Short-Term (6–12 months) | ❗ Mixed – Heavy losses from exceptional items may spook market, but Abbvie cash infusion and FDA recovery are strong positives. |
| Medium-Term (1–3 years) | ✅ Positive – Regulatory recovery should boost U.S. sales; cost optimization post-restructuring; potential for more IP monetization. |
| Valuation | Likely depressed due to one-time losses; actual operating EPS > ₹50/share annualized. Could be undervalued if turnaround holds. |
| Best Case | Glenmark replicates IGI model with new assets, wins U.S. approvals, deleverages. → Stock re-rating. |
| Worst Case | New FDA observations, more litigation, or poor R&D output → continuing volatility. |
💡 Final Conclusion
Glenmark is at an inflection point: - Massive headwinds from legacy litigation and inventory writedowns have caused short-term pain. - But strong tailwinds — the $700M AbbVie deal, FDA clearance, and strategic spin-offs/restructuring — position the company for recovery and potential transformation. - Primary growth avenue shifting from volume-driven generics to innovation-led licensing. - Investors should focus on cash flow, regulatory compliance, and next pipeline milestones rather than headline losses.
✅ Recommendation: Watchful optimism — a turnaround play with potential for outperformance if execution improves. Suitable for value + event-driven investors.
Let me know if you’d like a DCF valuation, peer comparison, or visual dashboard summary (PPT/Excel) based on the data.
Copyright © 2023 SAS Data Analytics Pvt. Ltd. All rights reserved.